Ch4 Polar Or Nonpolar Molecule Why are BF3, CF4, CO2, PF5, and SF6
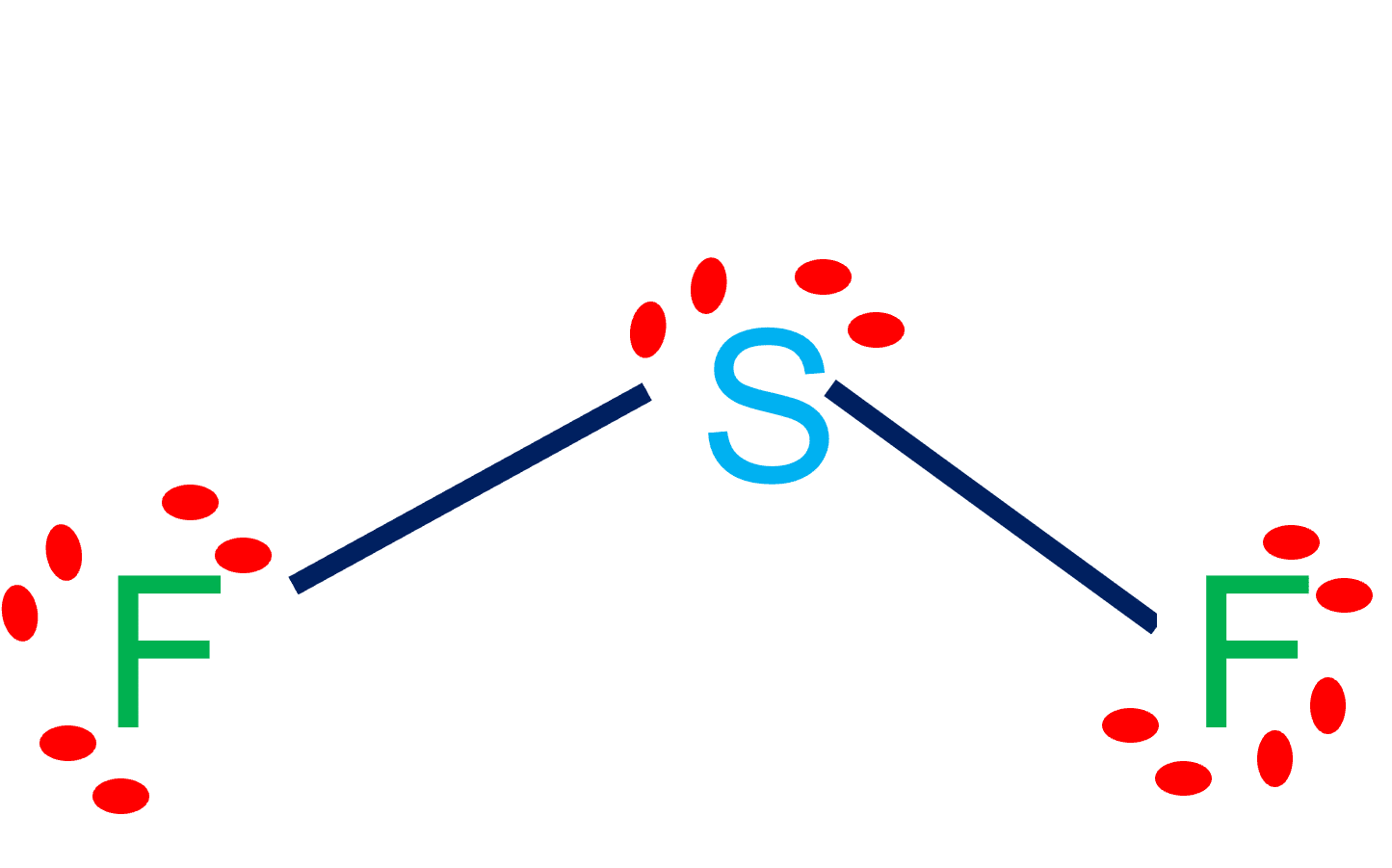
SF2 is polar in nature because the sulfur (2.58) and fluorine (3.98) atoms in the molecule differ in their electronegativity and the molecule has a bent geometrical shape. Therefore, the dipoles of the S-F bond do not cancel out each other and molecules turn out to be polar and contribute some dipole moment.

Polar and NonPolar Molecules YouTube
Explain how a molecule that contains polar bonds can be nonpolar. Answer. As long as the polar bonds are compensated (for example. two identical atoms are found directly across the central atom from one another), the molecule can be nonpolar. PROBLEM \(\PageIndex{2}\)

Polar vs. Nonpolar Bonds — Overview & Examples Expii Ionic Bonding
Choose the selection which correctly characterizes all three of the following substances in terms of whether they are polar or nonpolar: CS and Gere and IFS a) CS is polar and Gere is nonpolar and IFs is polar. b) CS is polar and GeHe is nonpolar and IFs is nonpolar. c) CS is polar and Gerais polar and IFs is nonpolar.
SF2 Lewis Structure & Molecular Geometry Simple Steps What's Insight
Page Contents show How to draw lewis structure of SF2? SF2 Lewis structure is made up of two atoms, sulfur (S), and fluorine (F), the sulfur (S) is in the central position and fluorine (F) atoms are on either side of it. The lewis structure of SF2 contains 16 nonbonding electrons and 4 bonding electrons.
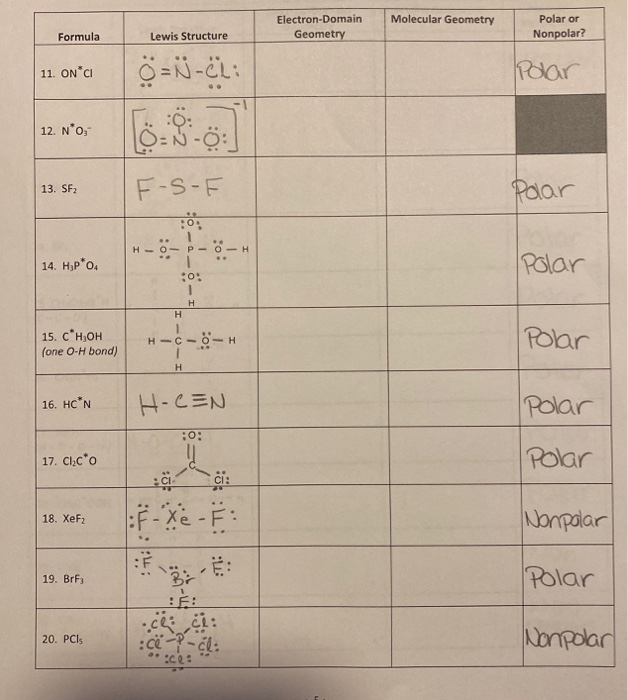
[Solved] image attached 1. Complete the table below. Indicate whether
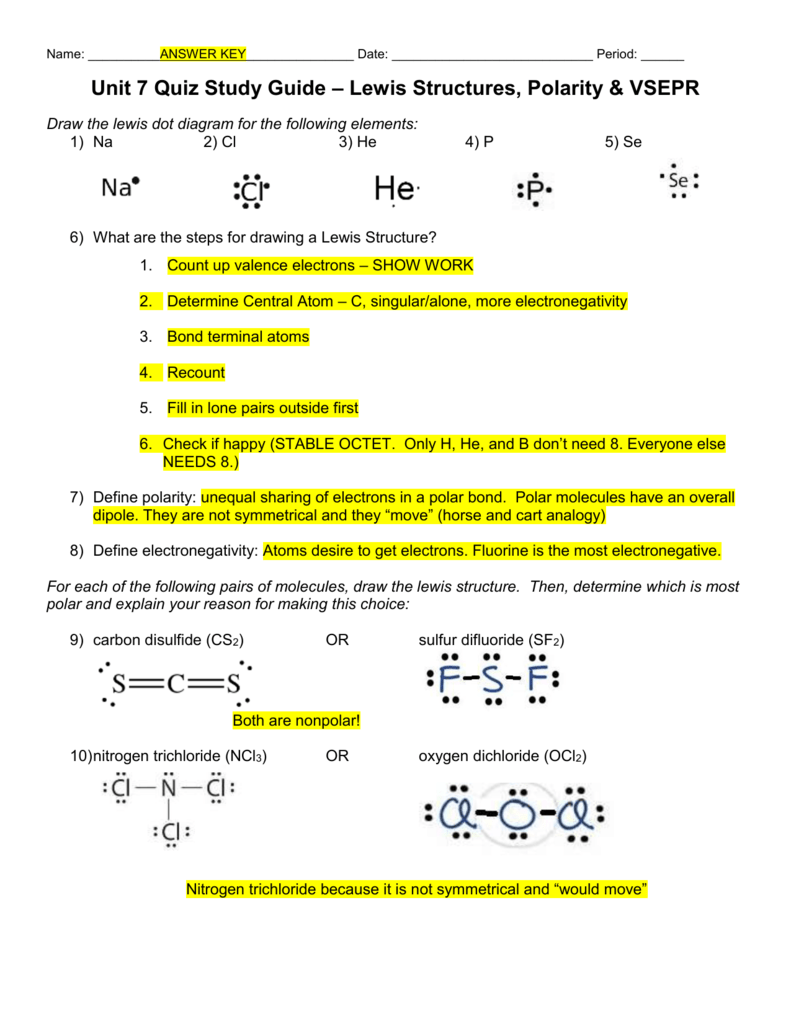
The molecule is symmetric. The two oxygen atoms pull on the electrons by exactly the same amount. Propane is nonpolar, because it is symmetric, with H atoms bonded to every side around the central atoms and no unshared pairs of electrons. Exercise 4.12. 1. Label each of the following as polar or nonpolar.
Is NO3 Polar or Nonpolar? (Nitrate ion) YouTube
Now in the next step we have to check whether these Se-F bonds are polar or nonpolar. And we also have to check the molecular geometry of SeF2. Step #2: Check whether individual bonds are polar or nonpolar. The chemical bonds can be either nonpolar, polar or ionic depending on the difference of the electronegativity values (ΔEN) between the.
Is Pcl5 Polar Or Nonpolar Asking List
0:00 / 1:58 Is SF4 (Sulfur tetrafluoride) Polar or Non-Polar? Wayne Breslyn 727K subscribers Subscribed 17K views 2 years ago Learn to determine if SF4 (Sulfur tetrafluoride) is polar or.
Is SF2 Polar or Nonpolar? YouTube
Have you ever wondered whether SF2 is a polar or nonpolar molecule? If so, you're not alone. This question has puzzled many chemistry students and professionals alike. In this article, we'll explore the properties of SF2 and determine once and for all whether it's polar or nonpolar. What is SF2?

Sf2 polar or nonpolar limfamike
(Explained in 3 Steps) SF2 is a polar molecule because it has poles of partial positive charge (ẟ+) and partial negative charge (ẟ-) on it. Let me explain this to you in 3 steps! Step #1: Draw the lewis structure Here is a skeleton of SF2 lewis structure and it contains two S-F bonds.
Sf2 polar or nonpolar limfath
Is the SF2 molecule polar or nonpolar? SF2 is a polar molecule because sulfur has extra electrons that push the molecule into a bent shape because of electron push. 7. What are the bond angles in the SF2 molecule? In the SF2 molecule, the angles between the bonds are approximately 98 degrees. This molecule has a bent or V-shaped molecular.

Is SF4 polar or nonpolar Science Education and Tutorials
Sulfur difluoride (SF2) is a polar molecule. The central sulfur (S) atom in SF2 is surrounded by two fluorine (F) atoms forming a bent or V-shaped molecule. A fluorine (F) atom is more electronegative than a sulfur (S) atom. Thus both S-F bonds are individually polar in the SF2 molecule and possess a specific dipole moment value.

Polare und unpolare Moleküle Free Press
Hey Guys!In this video, we are going to determine the polarity of Sulphur Difluoride having a chemical formula of SF2. It is made of one Sulphur atom and two.
Sf2 polar or nonpolar jujapress
Is SF2 Polar or Nonpolar? Wayne Breslyn 665K subscribers Subscribe 33K views 9 years ago If you look at the Lewis structure for SF2 might appear to be a symmetrical molecule. However, according.

Scl2 Polar Or Nonpolar Asking List
In SF2 lewis structure, the sulfur atom possesses two bonding pairs of electrons and two nonbonding pairs of electrons that reflect the VSEPR idea of AX2E2, which correlates to an angular/non-linear or bent molecular geometry. As a result, Sulfur Difluoride has a bent molecular geometry.

Is SF4 Polar or Nonpolar? (Sulfur Tetrafluoride) Math, Molecules
MakeTheBrainHappy Is SF2 Polar or Nonpolar? Answer: SF2 is a polar molecule due to the presence of lone pair electrons on sulfur which force the molecule to adopt a bent configuration due to electron-electron propulsion.
Sf2 polar or nonpolar lenaka
Best Answer. Copy. Think of the sulfite ion as a molecule with its geometry and dipole moment AND a net charge. The electron pair geometry is tetrahedral and the molecular geometry is trigonal.